- Home
- Resources
- Work samples
- Samples
- Expansion – AT
Science
Year 8
Satisfactory
Expansion in gases

 1
Annotation 1
1
Annotation 1
States key assumptions underlying the particle model of matter and lists key properties of gases
-
Annotations
-
1
Annotation 1
States key assumptions underlying the particle model of matter and lists key properties of gases
 1
Annotation 1
1
Annotation 1
Uses understanding of molecular kinetic theory to explain why gases expand and contract
-
Annotations
-
1
Annotation 1
Uses understanding of molecular kinetic theory to explain why gases expand and contract
 1
Annotation 1
1
Annotation 1
Identifies independent and dependent variables and describes how they are measured 2 Annotation 2
Attempts to identify controlled variables
-
Annotations
-
1
Annotation 1
Identifies independent and dependent variables and describes how they are measured -
2
Annotation 2
Attempts to identify controlled variables
 1
Annotation 1
1
Annotation 1
Considers safety concerns
-
Annotations
-
1
Annotation 1
Considers safety concerns

 1
Annotation 1
1
Annotation 1
Describes experimental procedure using clear step-by-step instructions
-
Annotations
-
1
Annotation 1
Describes experimental procedure using clear step-by-step instructions
 1
Annotation 1
1
Annotation 1
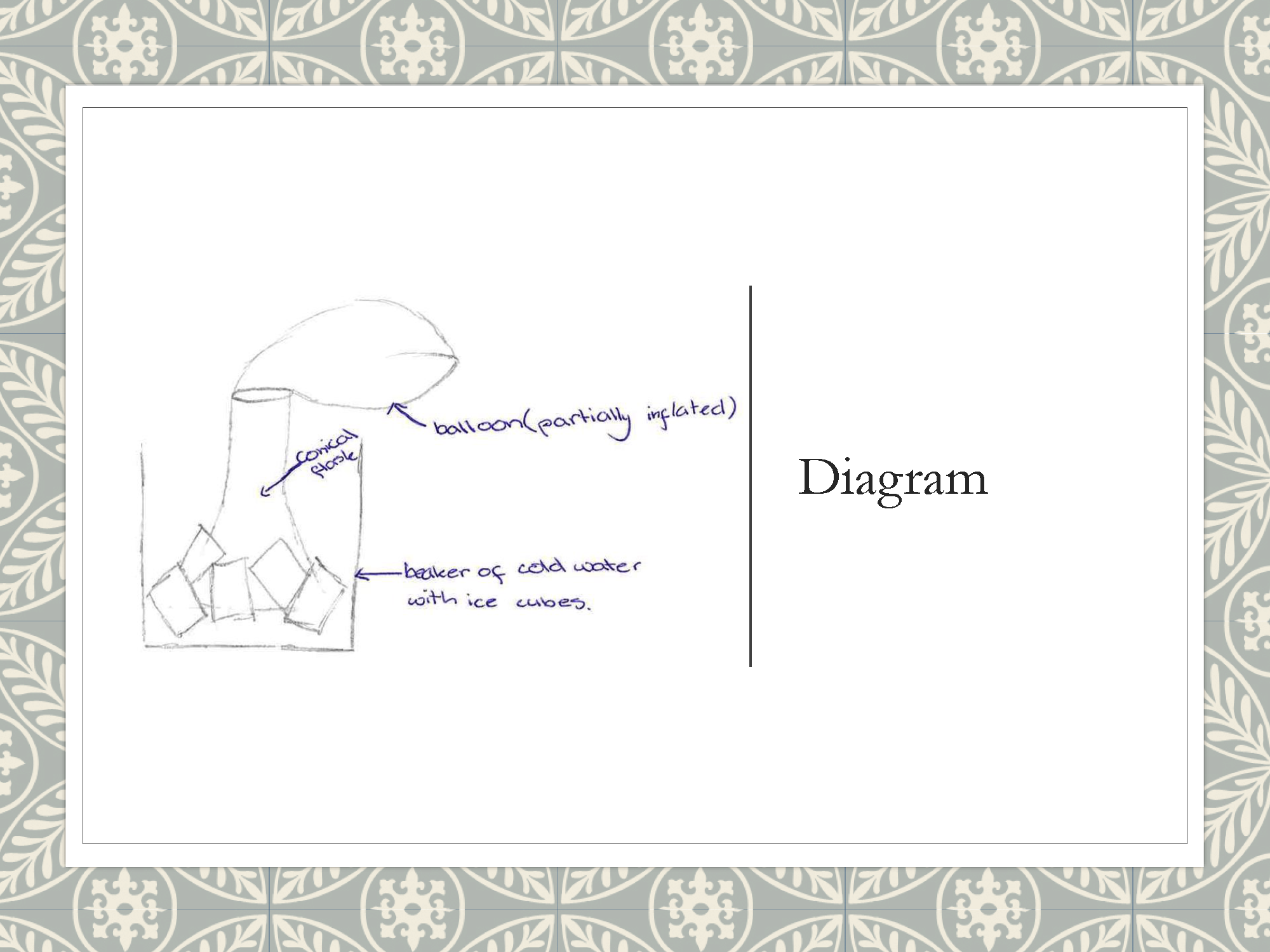
Creates annotated diagram to illustrate experimental setup
-
Annotations
-
1
Annotation 1
Creates annotated diagram to illustrate experimental setup
 1
Annotation 1
1
Annotation 1
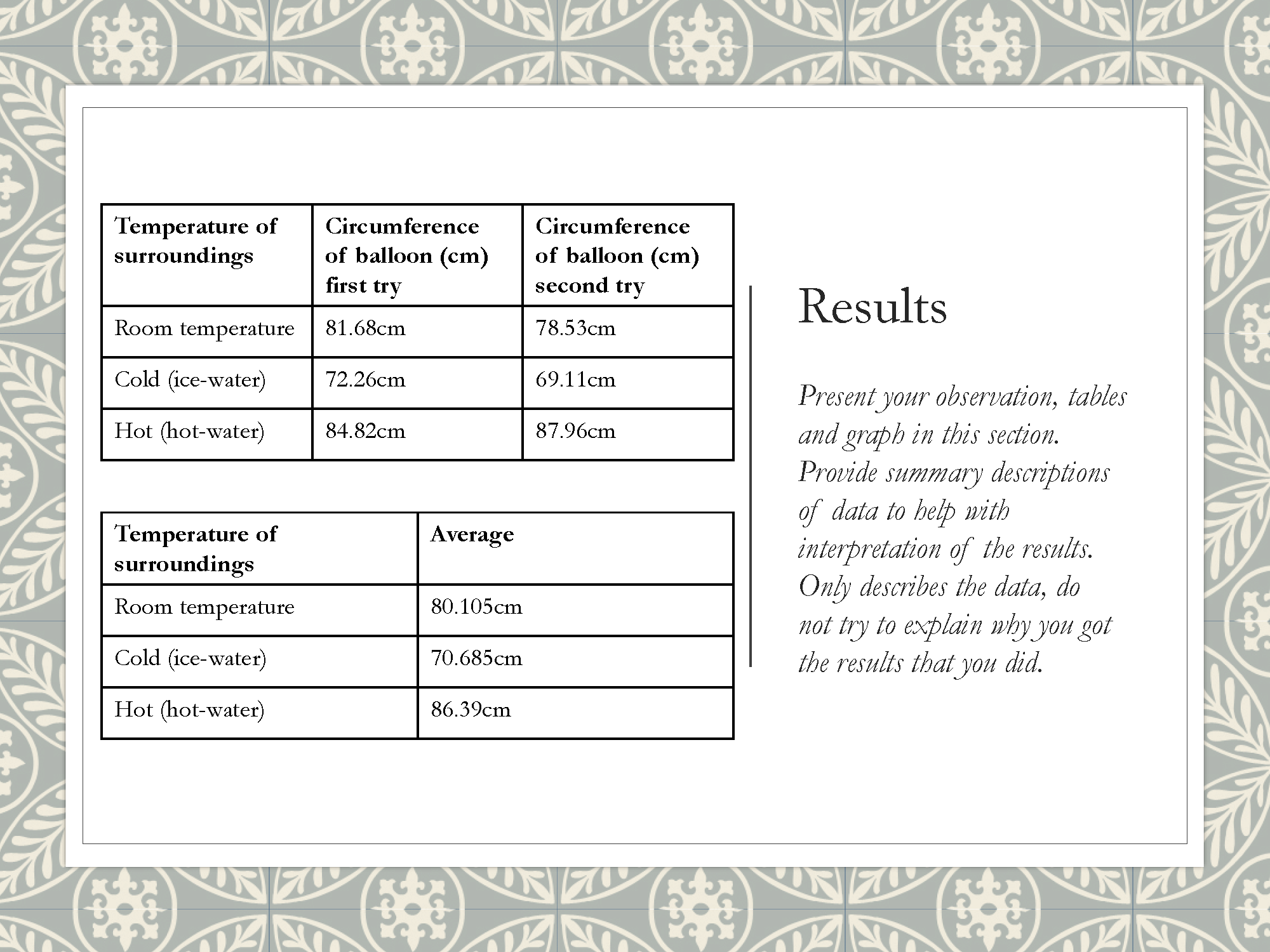
Displays results in table
-
Annotations
-
1
Annotation 1
Displays results in table
 1
Annotation 1
1
Annotation 1
Summarises findings and states conclusion
-
Annotations
-
1
Annotation 1
Summarises findings and states conclusion
 1
Annotation 1
1
Annotation 1
States real-life situation related to expansion of materials 2 Annotation 2
States experimental challenge and suggests improvement to method
-
Annotations
-
1
Annotation 1
States real-life situation related to expansion of materials -
2
Annotation 2
States experimental challenge and suggests improvement to method
Above satisfactory
Expansion in gases
 1
Annotation 1
1
Annotation 1
Uses understanding of molecular kinetic theory to explain why gases expand and names relevant scientific law 2 Annotation 2
Considers ethical and safety concerns 3 Annotation 3
Identifies independent and dependent variables and describes in detail how they are measured 4 Annotation 4
Identifies variables that need to be controlled and demonstrates understanding of molecular kinetic theory by including number of molecules 5 Annotation 5
Uses reasoning based on molecular kinetic theory to support hypothesis
-
Annotations
-
1
Annotation 1
Uses understanding of molecular kinetic theory to explain why gases expand and names relevant scientific law -
2
Annotation 2
Considers ethical and safety concerns -
3
Annotation 3
Identifies independent and dependent variables and describes in detail how they are measured -
4
Annotation 4
Identifies variables that need to be controlled and demonstrates understanding of molecular kinetic theory by including number of molecules -
5
Annotation 5
Uses reasoning based on molecular kinetic theory to support hypothesis
 1
Annotation 1
1
Annotation 1
Describes experimental procedure using clear step-by-step instructions 2 Annotation 2
Creates annotated diagram to illustrate experimental setup
-
Annotations
-
1
Annotation 1
Describes experimental procedure using clear step-by-step instructions -
2
Annotation 2
Creates annotated diagram to illustrate experimental setup
 1
Annotation 1
1
Annotation 1
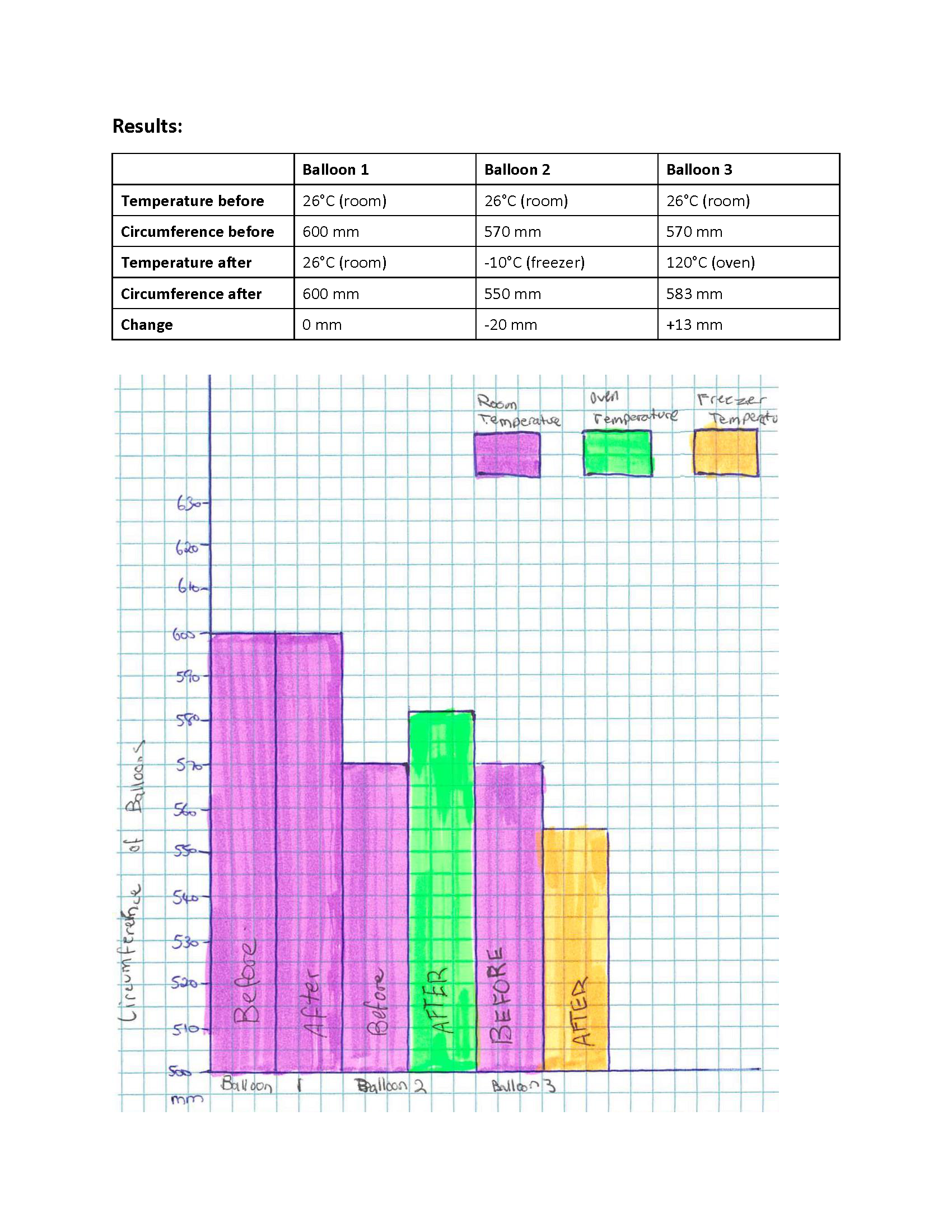
Displays results in table 2 Annotation 2
Creates column graph with appropriate axis titles and legend to display results
-
Annotations
-
1
Annotation 1
Displays results in table -
2
Annotation 2
Creates column graph with appropriate axis titles and legend to display results
 1
Annotation 1
1
Annotation 1
Refers to evidence to support conclusion 2 Annotation 2
Suggests improvements to experimental method 3 Annotation 3
Reflects on learning and applies it to describe real-life situation 4 Annotation 4
States relevant scientific law 5 Annotation 5
Uses molecular kinetic theory to explain results
-
Annotations
-
1
Annotation 1
Refers to evidence to support conclusion -
2
Annotation 2
Suggests improvements to experimental method -
3
Annotation 3
Reflects on learning and applies it to describe real-life situation -
4
Annotation 4
States relevant scientific law -
5
Annotation 5
Uses molecular kinetic theory to explain results
 1
Annotation 1
1
Annotation 1
Attempts to demonstrate Charles’s Law using correct mathematical approach
-
Annotations
-
1
Annotation 1
Attempts to demonstrate Charles’s Law using correct mathematical approach
 1
Annotation 1
1
Annotation 1
Identifies and formulates investigable question 2 Annotation 2
Researches relationship between temperature and volume and records key information including relevant scientific law 3 Annotation 3
Analyses potential ethical and safety concerns
-
Annotations
-
1
Annotation 1
Identifies and formulates investigable question -
2
Annotation 2
Researches relationship between temperature and volume and records key information including relevant scientific law -
3
Annotation 3
Analyses potential ethical and safety concerns
 1
Annotation 1
1
Annotation 1
Considers variables relevant to investigation 2 Annotation 2
Identifies independent variable and plans way to change it 3 Annotation 3
Identifies dependent variable and plans way to measure it 4 Annotation 4
Identifies key variable to be kept constant
-
Annotations
-
1
Annotation 1
Considers variables relevant to investigation -
2
Annotation 2
Identifies independent variable and plans way to change it -
3
Annotation 3
Identifies dependent variable and plans way to measure it -
4
Annotation 4
Identifies key variable to be kept constant
 1
Annotation 1
1
Annotation 1
Uses scientific reasoning based on molecular kinetic theory to develop hypothesis 2 Annotation 2
Formulates aim and predicts outcome of investigation
-
Annotations
-
1
Annotation 1
Uses scientific reasoning based on molecular kinetic theory to develop hypothesis -
2
Annotation 2
Formulates aim and predicts outcome of investigation
 1
Annotation 1
1
Annotation 1
Considers possible external influences on fairness of test 2 Annotation 2
Describes in detail experimental challenges and how they have been solved
-
Annotations
-
1
Annotation 1
Considers possible external influences on fairness of test -
2
Annotation 2
Describes in detail experimental challenges and how they have been solved
Satisfactory
Expansion in gases

 1
Annotation 1
1
Annotation 1
States key assumptions underlying the particle model of matter and lists key properties of gases
-
Annotations
-
1
Annotation 1
States key assumptions underlying the particle model of matter and lists key properties of gases
 1
Annotation 1
1
Annotation 1
Uses understanding of molecular kinetic theory to explain why gases expand and contract
-
Annotations
-
1
Annotation 1
Uses understanding of molecular kinetic theory to explain why gases expand and contract
 1
Annotation 1
1
Annotation 1
Identifies independent and dependent variables and describes how they are measured 2 Annotation 2
Attempts to identify controlled variables
-
Annotations
-
1
Annotation 1
Identifies independent and dependent variables and describes how they are measured -
2
Annotation 2
Attempts to identify controlled variables
 1
Annotation 1
1
Annotation 1
Considers safety concerns
-
Annotations
-
1
Annotation 1
Considers safety concerns

 1
Annotation 1
1
Annotation 1
Describes experimental procedure using clear step-by-step instructions
-
Annotations
-
1
Annotation 1
Describes experimental procedure using clear step-by-step instructions
 1
Annotation 1
1
Annotation 1
Creates annotated diagram to illustrate experimental setup
-
Annotations
-
1
Annotation 1
Creates annotated diagram to illustrate experimental setup
 1
Annotation 1
1
Annotation 1
Displays results in table
-
Annotations
-
1
Annotation 1
Displays results in table
 1
Annotation 1
1
Annotation 1
Summarises findings and states conclusion
-
Annotations
-
1
Annotation 1
Summarises findings and states conclusion
 1
Annotation 1
1
Annotation 1
States real-life situation related to expansion of materials 2 Annotation 2
States experimental challenge and suggests improvement to method
-
Annotations
-
1
Annotation 1
States real-life situation related to expansion of materials -
2
Annotation 2
States experimental challenge and suggests improvement to method