- Home
- Resources
- Work samples
- Samples
- Law of conservation of mass – AT
Science
Year 9
Satisfactory
Law of conservation of mass
 1
Annotation 1
1
Annotation 1
Sources definition of ‘chemical reaction’ from online source 2 Annotation 2
Explains why law of conservation of mass is important 3 Annotation 3
Describes selected chemical reactions, including balanced molecular equations and states symbols 4 Annotation 4
Describes use of one of the selected chemical reactions 5 Annotation 5
States reason for experiment 6 Annotation 6
Formulates hypothesis 7 Annotation 7
List steps of experimental procedure
-
Annotations
-
1
Annotation 1
Sources definition of ‘chemical reaction’ from online source -
2
Annotation 2
Explains why law of conservation of mass is important -
3
Annotation 3
Describes selected chemical reactions, including balanced molecular equations and states symbols -
4
Annotation 4
Describes use of one of the selected chemical reactions -
5
Annotation 5
States reason for experiment -
6
Annotation 6
Formulates hypothesis -
7
Annotation 7
List steps of experimental procedure
 1
Annotation 1
1
Annotation 1
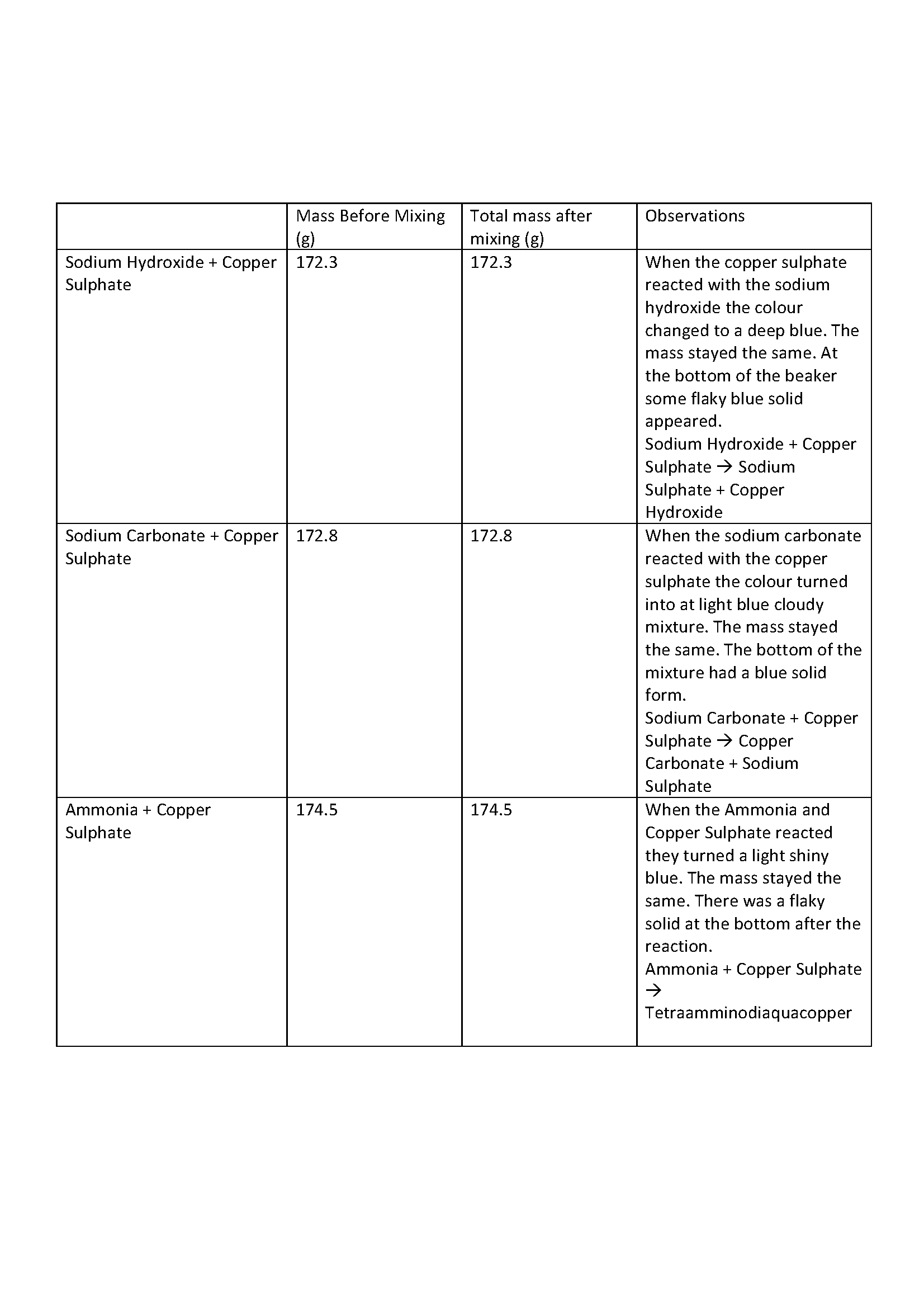
Presents measured data and detailed observations including word equations of chemical reactions in table
-
Annotations
-
1
Annotation 1
Presents measured data and detailed observations including word equations of chemical reactions in table
 1
Annotation 1
1
Annotation 1
Explains how experimental results support the law of conservation of mass 2 Annotation 2
Explains how a reaction involving production of a gas would not support the law of conservation of mass when conducted under same experimental conditions 3 Annotation 3
Argues that same reaction would support the law of conservation of mass if conducted in a closed system 4 Annotation 4
Reflects on understanding gained through conducting this experiment
-
Annotations
-
1
Annotation 1
Explains how experimental results support the law of conservation of mass -
2
Annotation 2
Explains how a reaction involving production of a gas would not support the law of conservation of mass when conducted under same experimental conditions -
3
Annotation 3
Argues that same reaction would support the law of conservation of mass if conducted in a closed system -
4
Annotation 4
Reflects on understanding gained through conducting this experiment
Above satisfactory
Law of conservation of mass
 1
Annotation 1
1
Annotation 1
Explains chemical reactions in terms of electron rearrangements 2 Annotation 2
Describes occurrence, use and importance of selected chemical reactions, including balanced molecular equations 3 Annotation 3
Explains and demonstrates how chemical equations are balanced using colour coding to aid readers’ comprehension 4 Annotation 4
States law of conservation of mass including type of system it applies to 5 Annotation 5
Explains why law of conservation of mass is important 6 Annotation 6
States reason for experiment 7 Annotation 7
Outlines how experiment will be conducted
-
Annotations
-
1
Annotation 1
Explains chemical reactions in terms of electron rearrangements -
2
Annotation 2
Describes occurrence, use and importance of selected chemical reactions, including balanced molecular equations -
3
Annotation 3
Explains and demonstrates how chemical equations are balanced using colour coding to aid readers’ comprehension -
4
Annotation 4
States law of conservation of mass including type of system it applies to -
5
Annotation 5
Explains why law of conservation of mass is important -
6
Annotation 6
States reason for experiment -
7
Annotation 7
Outlines how experiment will be conducted
 1
Annotation 1
1
Annotation 1
Formulates hypothesis 2 Annotation 2
Describes safety precautions undertaken 3 Annotation 3
Describes experimental procedure
-
Annotations
-
1
Annotation 1
Formulates hypothesis -
2
Annotation 2
Describes safety precautions undertaken -
3
Annotation 3
Describes experimental procedure
 1
Annotation 1
1
Annotation 1
Presents measured data and detailed observations in table 2 Annotation 2
Provides photographic evidence of reactions
-
Annotations
-
1
Annotation 1
Presents measured data and detailed observations in table -
2
Annotation 2
Provides photographic evidence of reactions
 1
Annotation 1
1
Annotation 1
Identifies colour change as evidence for chemical reaction 2 Annotation 2
Summarises experimental results 3 Annotation 3
Reflects critically on experimental setup and correctly concludes that law of conservation of mass cannot be demonstrated conclusively by this experiment 4 Annotation 4
Explains how a reaction involving production of a gas would not support law of conservation of mass when conducted in an open system 5 Annotation 5
Concludes that experiment was not suitable to confirm law of conservation of mass 6 Annotation 6
Reflects on understanding gained through conducting this experiment
-
Annotations
-
1
Annotation 1
Identifies colour change as evidence for chemical reaction -
2
Annotation 2
Summarises experimental results -
3
Annotation 3
Reflects critically on experimental setup and correctly concludes that law of conservation of mass cannot be demonstrated conclusively by this experiment -
4
Annotation 4
Explains how a reaction involving production of a gas would not support law of conservation of mass when conducted in an open system -
5
Annotation 5
Concludes that experiment was not suitable to confirm law of conservation of mass -
6
Annotation 6
Reflects on understanding gained through conducting this experiment
 1
Annotation 1
1
Annotation 1
Provides list of resources used to research topic
-
Annotations
-
1
Annotation 1
Provides list of resources used to research topic
Satisfactory
Law of conservation of mass
 1
Annotation 1
1
Annotation 1
Sources definition of ‘chemical reaction’ from online source 2 Annotation 2
Explains why law of conservation of mass is important 3 Annotation 3
Describes selected chemical reactions, including balanced molecular equations and states symbols 4 Annotation 4
Describes use of one of the selected chemical reactions 5 Annotation 5
States reason for experiment 6 Annotation 6
Formulates hypothesis 7 Annotation 7
List steps of experimental procedure
-
Annotations
-
1
Annotation 1
Sources definition of ‘chemical reaction’ from online source -
2
Annotation 2
Explains why law of conservation of mass is important -
3
Annotation 3
Describes selected chemical reactions, including balanced molecular equations and states symbols -
4
Annotation 4
Describes use of one of the selected chemical reactions -
5
Annotation 5
States reason for experiment -
6
Annotation 6
Formulates hypothesis -
7
Annotation 7
List steps of experimental procedure
 1
Annotation 1
1
Annotation 1
Presents measured data and detailed observations including word equations of chemical reactions in table
-
Annotations
-
1
Annotation 1
Presents measured data and detailed observations including word equations of chemical reactions in table
 1
Annotation 1
1
Annotation 1
Explains how experimental results support the law of conservation of mass 2 Annotation 2
Explains how a reaction involving production of a gas would not support the law of conservation of mass when conducted under same experimental conditions 3 Annotation 3
Argues that same reaction would support the law of conservation of mass if conducted in a closed system 4 Annotation 4
Reflects on understanding gained through conducting this experiment
-
Annotations
-
1
Annotation 1
Explains how experimental results support the law of conservation of mass -
2
Annotation 2
Explains how a reaction involving production of a gas would not support the law of conservation of mass when conducted under same experimental conditions -
3
Annotation 3
Argues that same reaction would support the law of conservation of mass if conducted in a closed system -
4
Annotation 4
Reflects on understanding gained through conducting this experiment