- Home
- Resources
- Work samples
- Samples
- Periodic table pamphlet – ABOVE
Science
Year 10
Above satisfactory
How to use the Periodic Table
Annotations overview
Uses fundamental questions as headings throughout document, consistent with its purpose and target audience
 1
Annotation 1
1
Annotation 1
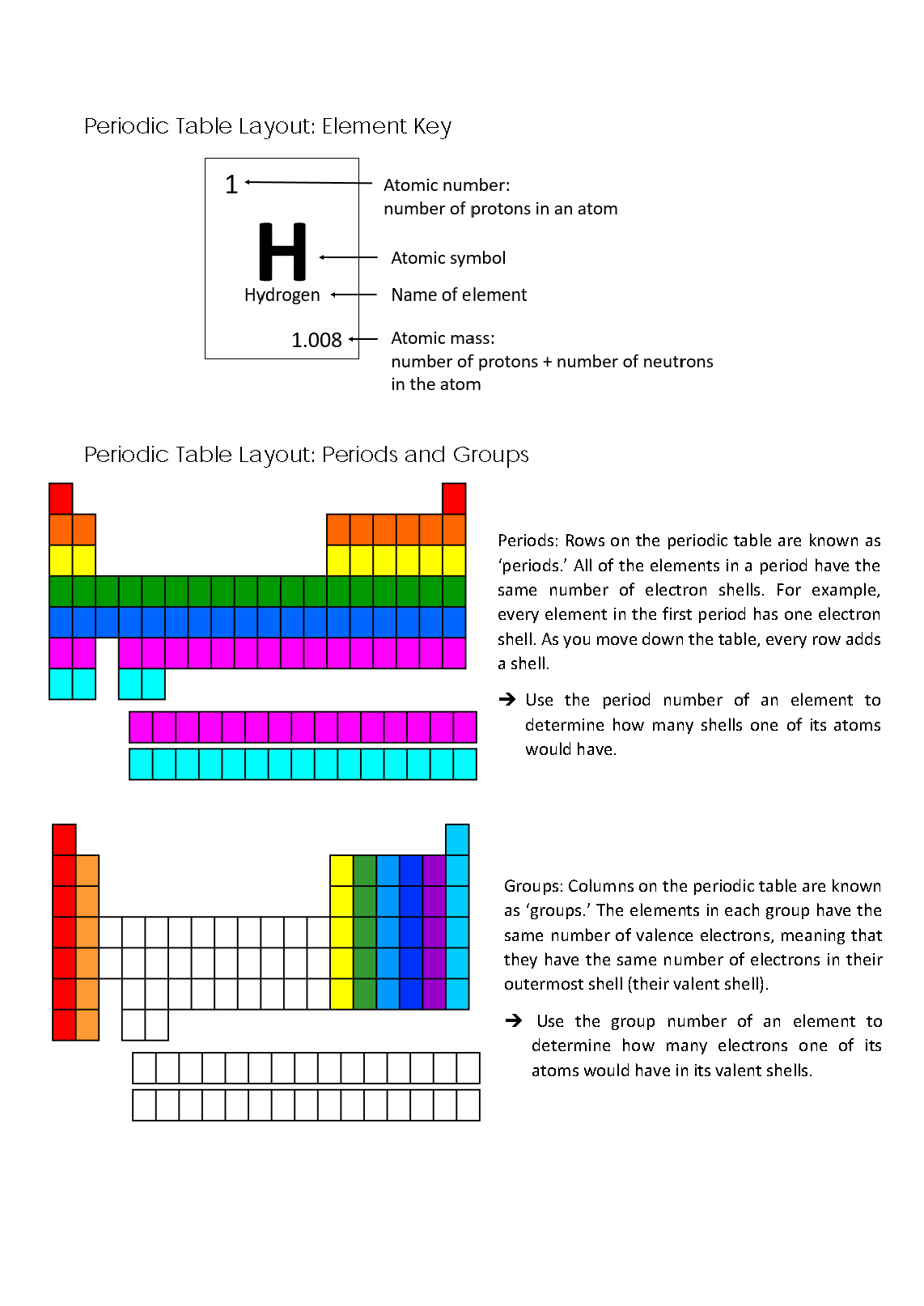
Provides succinct definition of the Periodic Table, demonstrates understanding of relationship between atomic mass and recurring properties of elements 2 Annotation 2
Creates timeline of key discoveries that shaped our understanding of elements and their atomic structures
-
Annotations
-
1
Annotation 1
Provides succinct definition of the Periodic Table, demonstrates understanding of relationship between atomic mass and recurring properties of elements -
2
Annotation 2
Creates timeline of key discoveries that shaped our understanding of elements and their atomic structures
 1
Annotation 1
1
Annotation 1
Explains how an element’s period number is linked to its electron shell structure 2 Annotation 2
Explains how an element’s group number is linked to its number of valence electrons
-
Annotations
-
1
Annotation 1
Explains how an element’s period number is linked to its electron shell structure -
2
Annotation 2
Explains how an element’s group number is linked to its number of valence electrons
 1
Annotation 1
1
Annotation 1
Defines key scientific terms 2 Annotation 2
Understands that an element is defined by the number of protons in its nucleus 3 Annotation 3
Displays annotated diagram of internal structure of an atom and provides definitions and key physical data for its components 4 Annotation 4
Provides images highlighting major groups of elements
-
Annotations
-
1
Annotation 1
Defines key scientific terms -
2
Annotation 2
Understands that an element is defined by the number of protons in its nucleus -
3
Annotation 3
Displays annotated diagram of internal structure of an atom and provides definitions and key physical data for its components -
4
Annotation 4
Provides images highlighting major groups of elements
 1
Annotation 1
1
Annotation 1
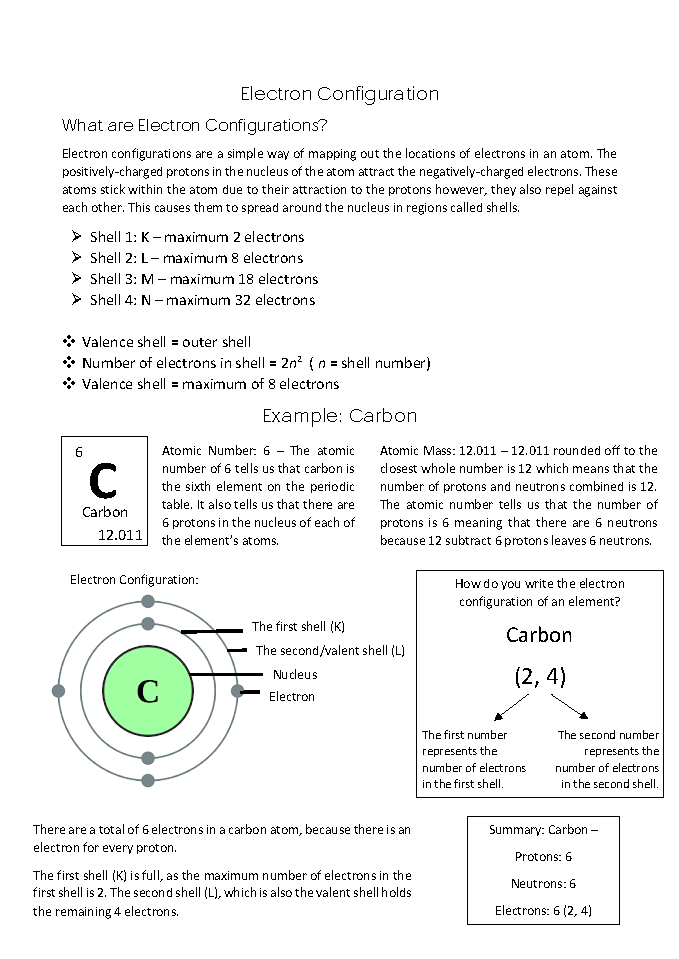
Defines electron configuration based on its purpose 2 Annotation 2
Understands balance of attractive and repulsive forces between protons and electrons as underlying atom’s stability and internal structure 3 Annotation 3
Understands shells as regions rather than orbits 4 Annotation 4
Lists key data and naming conventions for electronic shells 5 Annotation 5
Displays mathematical relationship between shell number and its maximum occupancy 6 Annotation 6
Explains in detail how to interpret information given for an element in Periodic Table 7 Annotation 7
Gives detailed example of how to determine and display an element’s electron configuration
-
Annotations
-
1
Annotation 1
Defines electron configuration based on its purpose -
2
Annotation 2
Understands balance of attractive and repulsive forces between protons and electrons as underlying atom’s stability and internal structure -
3
Annotation 3
Understands shells as regions rather than orbits -
4
Annotation 4
Lists key data and naming conventions for electronic shells -
5
Annotation 5
Displays mathematical relationship between shell number and its maximum occupancy -
6
Annotation 6
Explains in detail how to interpret information given for an element in Periodic Table -
7
Annotation 7
Gives detailed example of how to determine and display an element’s electron configuration
 1
Annotation 1
1
Annotation 1
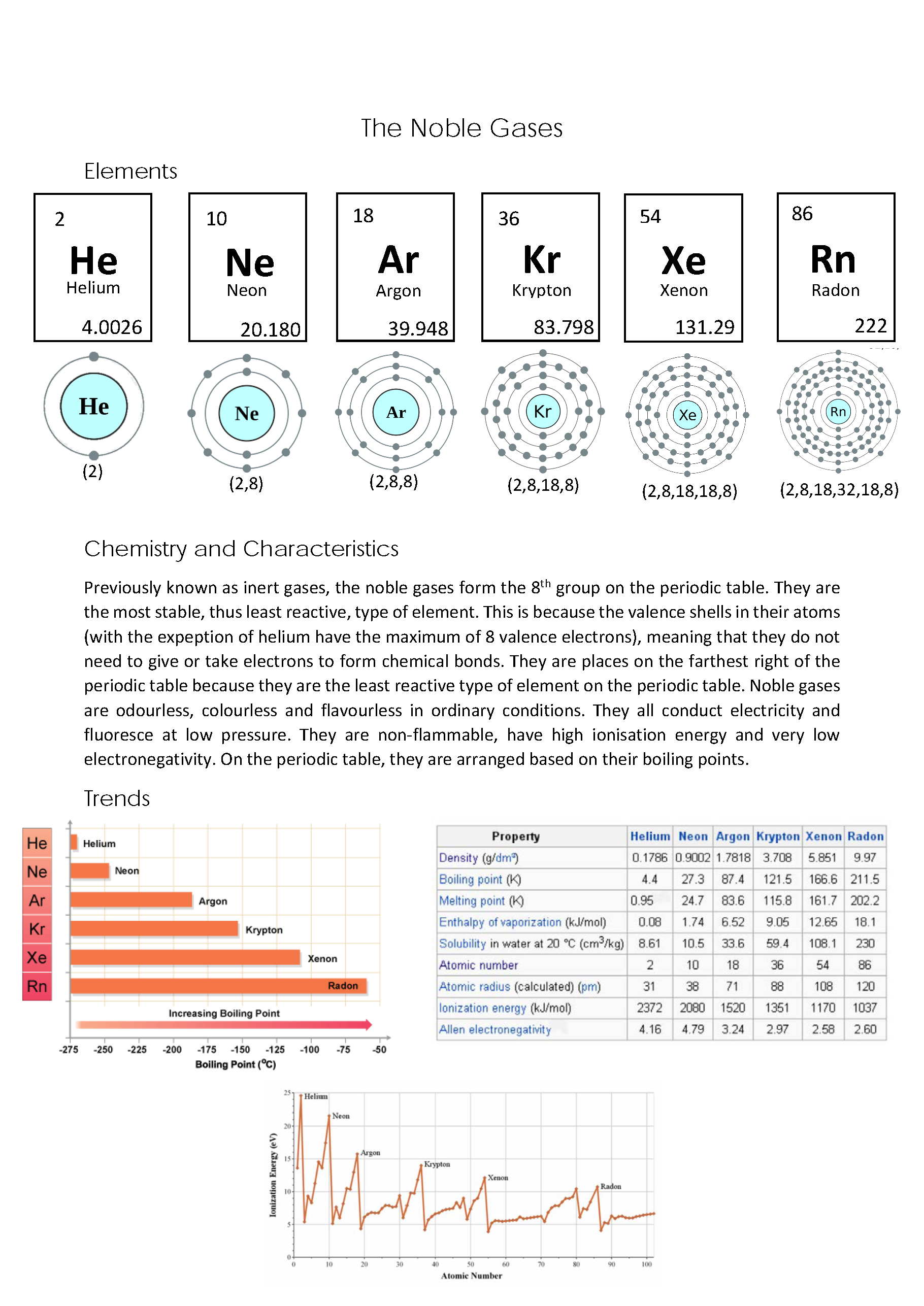
Explains causal link between low reactivity of noble gas elements and their electronic structures 2 Annotation 2
Lists common key properties of noble gas elements consistent with their low reactivities 3 Annotation 3
Displays data table of selected physical properties of noble gases demonstrating trends within group 4 Annotation 4
Displays graph demonstrating exceptional status of noble gases among elements
-
Annotations
-
1
Annotation 1
Explains causal link between low reactivity of noble gas elements and their electronic structures -
2
Annotation 2
Lists common key properties of noble gas elements consistent with their low reactivities -
3
Annotation 3
Displays data table of selected physical properties of noble gases demonstrating trends within group -
4
Annotation 4
Displays graph demonstrating exceptional status of noble gases among elements
 1
Annotation 1
1
Annotation 1
Describes histories of discovery and historical and modern uses of selected noble gas elements
-
Annotations
-
1
Annotation 1
Describes histories of discovery and historical and modern uses of selected noble gas elements
 1
Annotation 1
1
Annotation 1
Provides list of appropriate sources consulted during investigation
-
Annotations
-
1
Annotation 1
Provides list of appropriate sources consulted during investigation
Satisfactory
How to use the Periodic Table
 1
Annotation 1
1
Annotation 1
Gives high-level introduction to Periodic Table
-
Annotations
-
1
Annotation 1
Gives high-level introduction to Periodic Table
 1
Annotation 1
1
Annotation 1
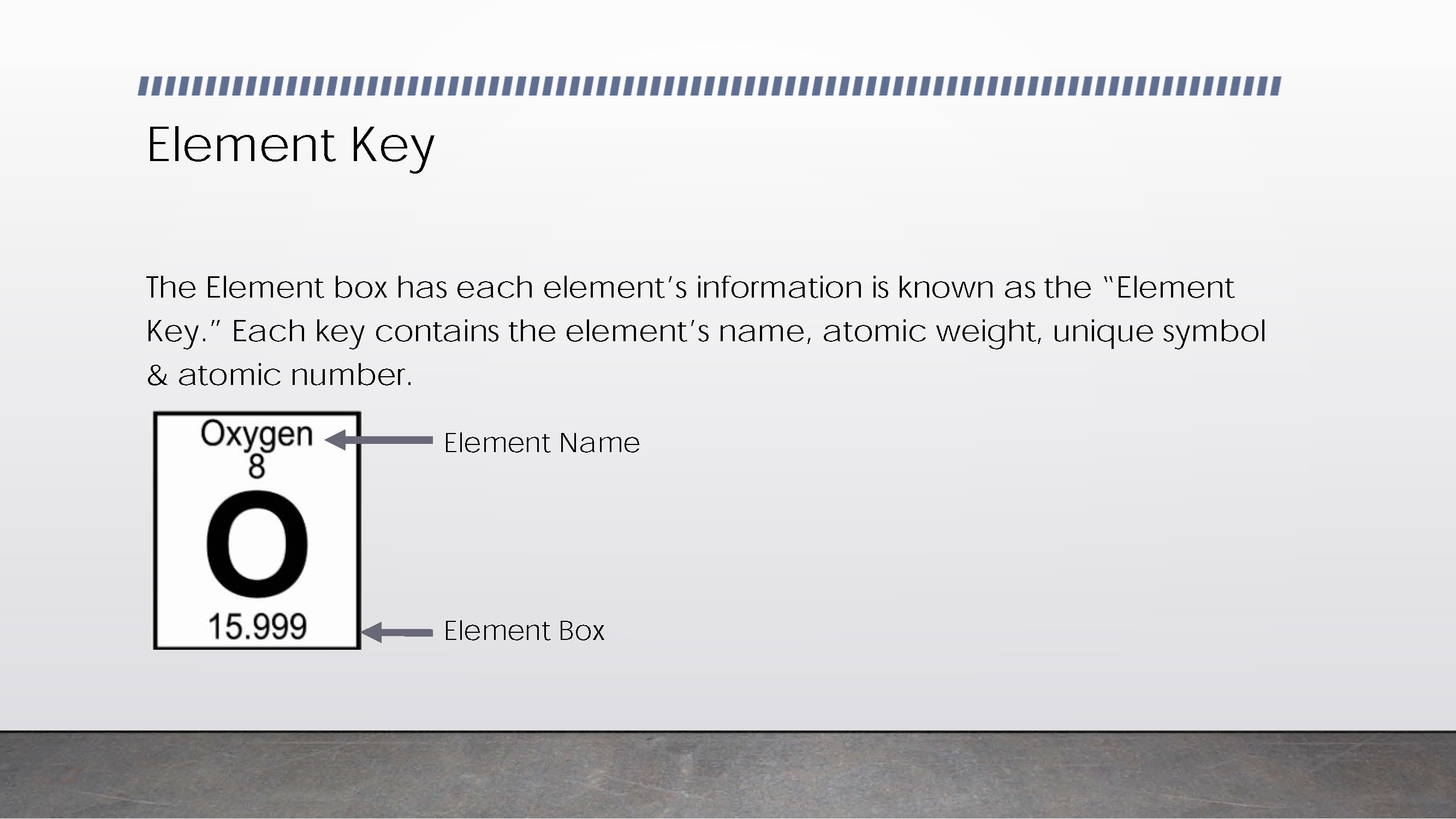
Explains and displays key to read information about elements
-
Annotations
-
1
Annotation 1
Explains and displays key to read information about elements
 1
Annotation 1
1
Annotation 1
States connection between atomic number of an element, its number of protons and electrons, and its position in table
-
Annotations
-
1
Annotation 1
States connection between atomic number of an element, its number of protons and electrons, and its position in table
 1
Annotation 1
1
Annotation 1
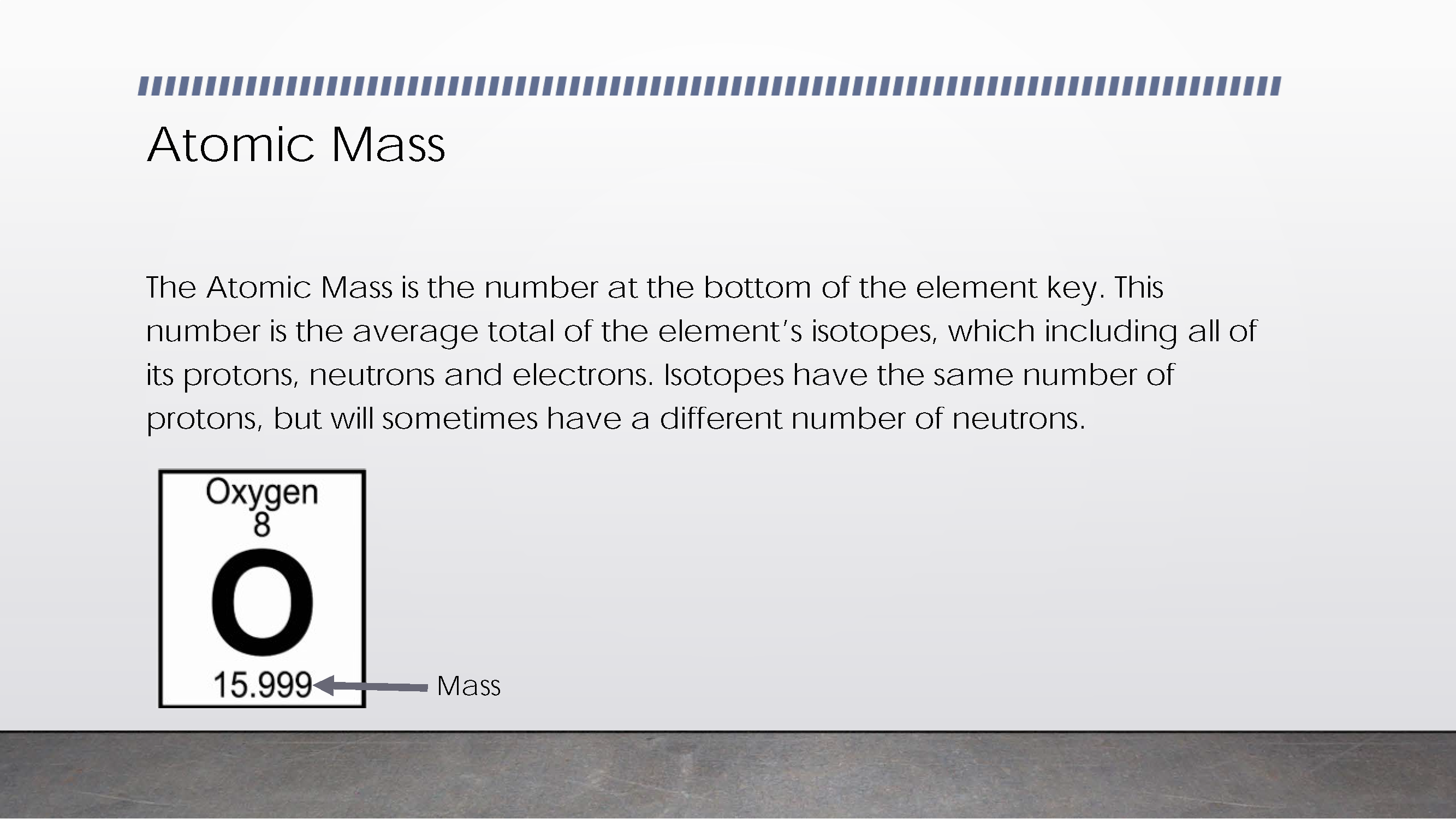
Demonstrates understanding of element’s atomic mass as average of its isotopes 2 Annotation 2
Explains what isotopes are
-
Annotations
-
1
Annotation 1
Demonstrates understanding of element’s atomic mass as average of its isotopes -
2
Annotation 2
Explains what isotopes are
 1
Annotation 1
1
Annotation 1
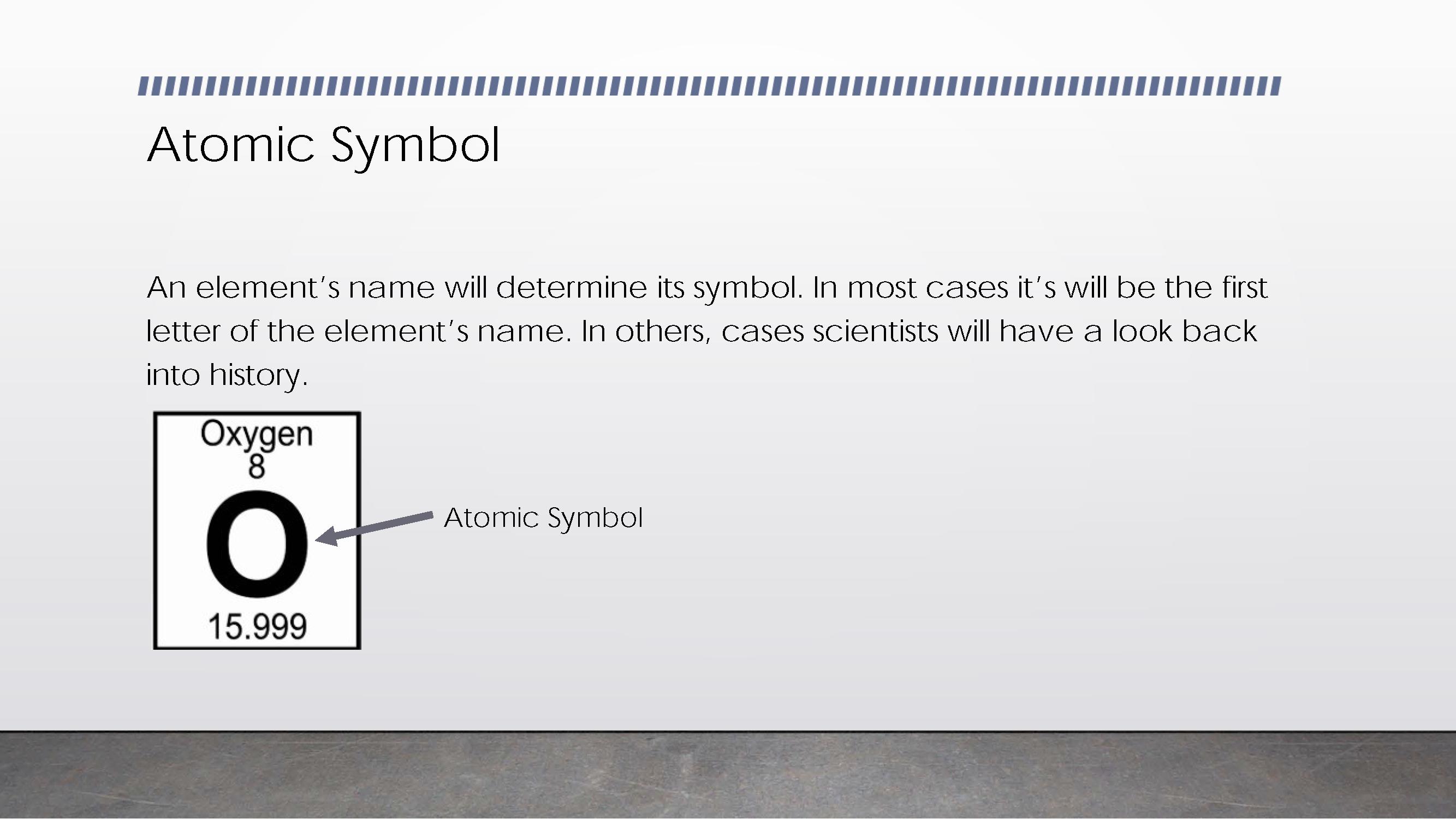
States how atomic symbol is derived
-
Annotations
-
1
Annotation 1
States how atomic symbol is derived
 1
Annotation 1
1
Annotation 1
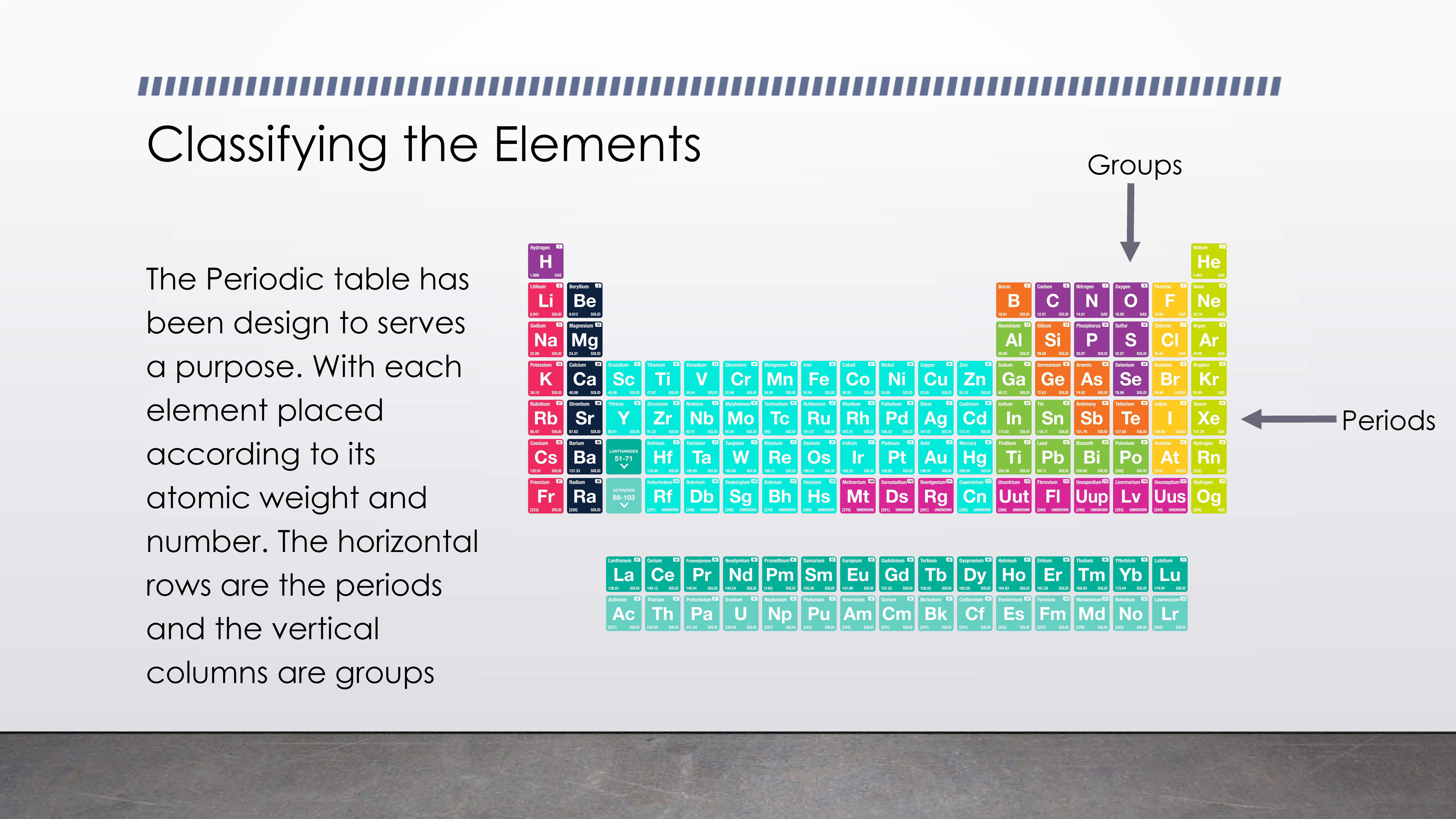
Supports explanation of terms ‘group’ and ‘period’ with visual aids
-
Annotations
-
1
Annotation 1
Supports explanation of terms ‘group’ and ‘period’ with visual aids
 1
Annotation 1
1
Annotation 1
Relates chemical properties of elements to their position in columns (groups) 2 Annotation 2
Attempts to link element’s group position to its electronic structure 3 Annotation 3
Demonstrates confusion around the use of the term ‘group’ 4 Annotation 4
Describes common key properties of two groups of metals
-
Annotations
-
1
Annotation 1
Relates chemical properties of elements to their position in columns (groups) -
2
Annotation 2
Attempts to link element’s group position to its electronic structure -
3
Annotation 3
Demonstrates confusion around the use of the term ‘group’ -
4
Annotation 4
Describes common key properties of two groups of metals
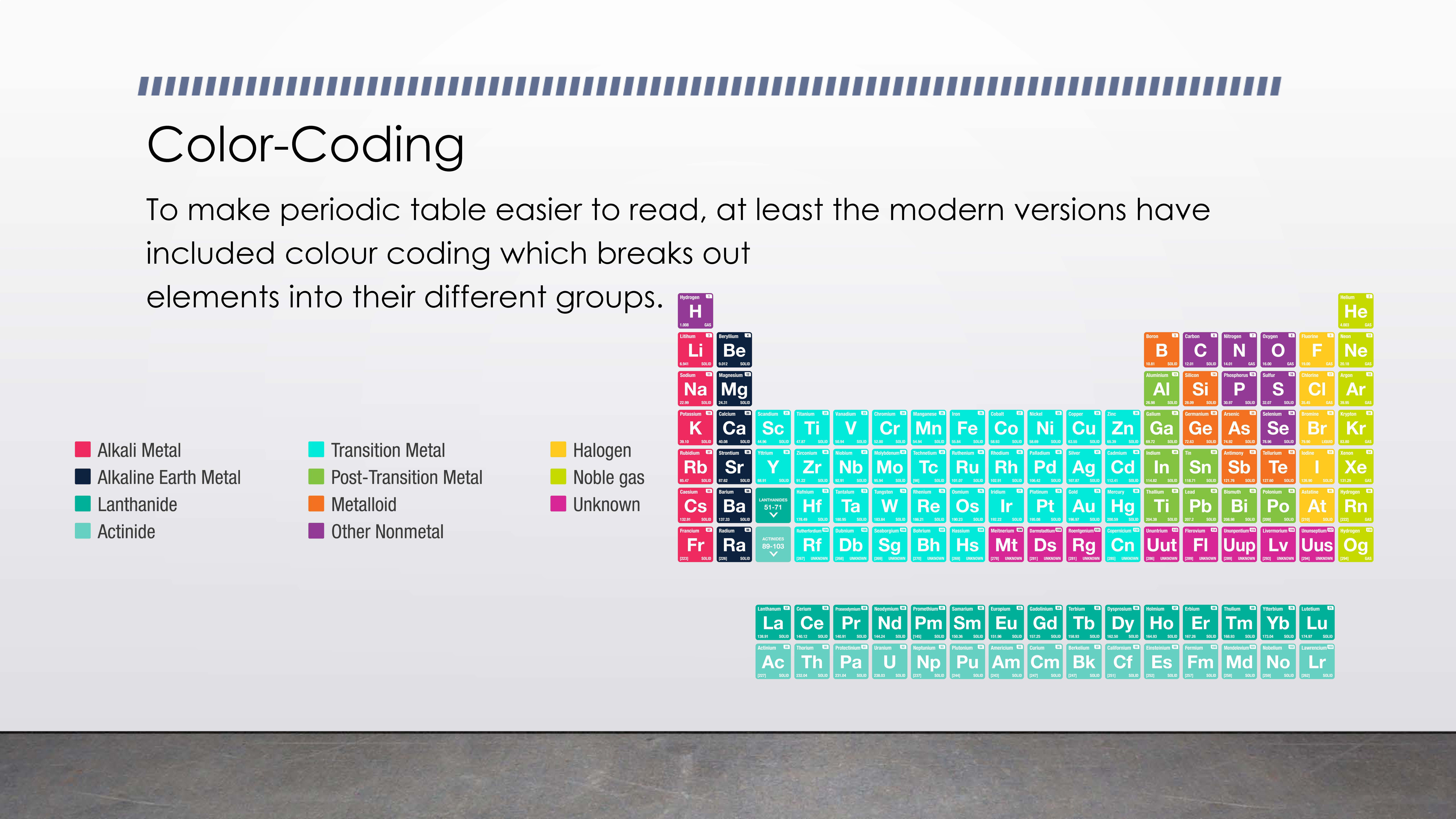 1
Annotation 1
1
Annotation 1
Describes how colour-coding links to grouping of elements and helps communicate organisation of the Periodic Table
-
Annotations
-
1
Annotation 1
Describes how colour-coding links to grouping of elements and helps communicate organisation of the Periodic Table
 1
Annotation 1
1
Annotation 1
Describes positional partition between metal and non-metal elements in Periodic Table 2 Annotation 2
Describes general trends for reactivity of (metal) elements within group and period 3 Annotation 3
Draws logical conclusion from described trends and identifies most reactive (metal) element based on its position
-
Annotations
-
1
Annotation 1
Describes positional partition between metal and non-metal elements in Periodic Table -
2
Annotation 2
Describes general trends for reactivity of (metal) elements within group and period -
3
Annotation 3
Draws logical conclusion from described trends and identifies most reactive (metal) element based on its position
 1
Annotation 1
1
Annotation 1
Describes important and interesting real-world applications of selected elements
-
Annotations
-
1
Annotation 1
Describes important and interesting real-world applications of selected elements
 1
Annotation 1
1
Annotation 1
Provides succinct definition for ions including explanation of the term ‘cation’
-
Annotations
-
1
Annotation 1
Provides succinct definition for ions including explanation of the term ‘cation’

 1
Annotation 1
1
Annotation 1
Provides list of sources used to research the topic
-
Annotations
-
1
Annotation 1
Provides list of sources used to research the topic
Above satisfactory
How to use the Periodic Table
Annotations overview
Uses fundamental questions as headings throughout document, consistent with its purpose and target audience
 1
Annotation 1
1
Annotation 1
Provides succinct definition of the Periodic Table, demonstrates understanding of relationship between atomic mass and recurring properties of elements 2 Annotation 2
Creates timeline of key discoveries that shaped our understanding of elements and their atomic structures
-
Annotations
-
1
Annotation 1
Provides succinct definition of the Periodic Table, demonstrates understanding of relationship between atomic mass and recurring properties of elements -
2
Annotation 2
Creates timeline of key discoveries that shaped our understanding of elements and their atomic structures
 1
Annotation 1
1
Annotation 1
Explains how an element’s period number is linked to its electron shell structure 2 Annotation 2
Explains how an element’s group number is linked to its number of valence electrons
-
Annotations
-
1
Annotation 1
Explains how an element’s period number is linked to its electron shell structure -
2
Annotation 2
Explains how an element’s group number is linked to its number of valence electrons
 1
Annotation 1
1
Annotation 1
Defines key scientific terms 2 Annotation 2
Understands that an element is defined by the number of protons in its nucleus 3 Annotation 3
Displays annotated diagram of internal structure of an atom and provides definitions and key physical data for its components 4 Annotation 4
Provides images highlighting major groups of elements
-
Annotations
-
1
Annotation 1
Defines key scientific terms -
2
Annotation 2
Understands that an element is defined by the number of protons in its nucleus -
3
Annotation 3
Displays annotated diagram of internal structure of an atom and provides definitions and key physical data for its components -
4
Annotation 4
Provides images highlighting major groups of elements
 1
Annotation 1
1
Annotation 1
Defines electron configuration based on its purpose 2 Annotation 2
Understands balance of attractive and repulsive forces between protons and electrons as underlying atom’s stability and internal structure 3 Annotation 3
Understands shells as regions rather than orbits 4 Annotation 4
Lists key data and naming conventions for electronic shells 5 Annotation 5
Displays mathematical relationship between shell number and its maximum occupancy 6 Annotation 6
Explains in detail how to interpret information given for an element in Periodic Table 7 Annotation 7
Gives detailed example of how to determine and display an element’s electron configuration
-
Annotations
-
1
Annotation 1
Defines electron configuration based on its purpose -
2
Annotation 2
Understands balance of attractive and repulsive forces between protons and electrons as underlying atom’s stability and internal structure -
3
Annotation 3
Understands shells as regions rather than orbits -
4
Annotation 4
Lists key data and naming conventions for electronic shells -
5
Annotation 5
Displays mathematical relationship between shell number and its maximum occupancy -
6
Annotation 6
Explains in detail how to interpret information given for an element in Periodic Table -
7
Annotation 7
Gives detailed example of how to determine and display an element’s electron configuration
 1
Annotation 1
1
Annotation 1
Explains causal link between low reactivity of noble gas elements and their electronic structures 2 Annotation 2
Lists common key properties of noble gas elements consistent with their low reactivities 3 Annotation 3
Displays data table of selected physical properties of noble gases demonstrating trends within group 4 Annotation 4
Displays graph demonstrating exceptional status of noble gases among elements
-
Annotations
-
1
Annotation 1
Explains causal link between low reactivity of noble gas elements and their electronic structures -
2
Annotation 2
Lists common key properties of noble gas elements consistent with their low reactivities -
3
Annotation 3
Displays data table of selected physical properties of noble gases demonstrating trends within group -
4
Annotation 4
Displays graph demonstrating exceptional status of noble gases among elements
 1
Annotation 1
1
Annotation 1
Describes histories of discovery and historical and modern uses of selected noble gas elements
-
Annotations
-
1
Annotation 1
Describes histories of discovery and historical and modern uses of selected noble gas elements
 1
Annotation 1
1
Annotation 1
Provides list of appropriate sources consulted during investigation
-
Annotations
-
1
Annotation 1
Provides list of appropriate sources consulted during investigation