- Home
- Resources
- Work samples
- Samples
- Rates of reaction – AT
Science
Year 10
Satisfactory
Rates of reaction
 1
Annotation 1
1
Annotation 1
States principal hypothesis of collision theory 2 Annotation 2
Identifies factors that influence rate of chemical reactions, including temperature, concentration and surface area 3 Annotation 3
Uses mathematical reasoning to explain effect of concentration on reaction rate 4 Annotation 4
Outlines experimental design specifying acid concentration as independent variable 5 Annotation 5
Makes prediction that is consistent with collision theory while demonstrating misconception about nature of ‘evidence’ 6 Annotation 6
Designs appropriate investigation method 7 Annotation 7
Considers reliability by performing repeated trials 8 Annotation 8
Considers safety precautions by specifying protective clothing and eyewear
-
Annotations
-
1
Annotation 1
States principal hypothesis of collision theory -
2
Annotation 2
Identifies factors that influence rate of chemical reactions, including temperature, concentration and surface area -
3
Annotation 3
Uses mathematical reasoning to explain effect of concentration on reaction rate -
4
Annotation 4
Outlines experimental design specifying acid concentration as independent variable -
5
Annotation 5
Makes prediction that is consistent with collision theory while demonstrating misconception about nature of ‘evidence’ -
6
Annotation 6
Designs appropriate investigation method -
7
Annotation 7
Considers reliability by performing repeated trials -
8
Annotation 8
Considers safety precautions by specifying protective clothing and eyewear
 1
Annotation 1
1
Annotation 1
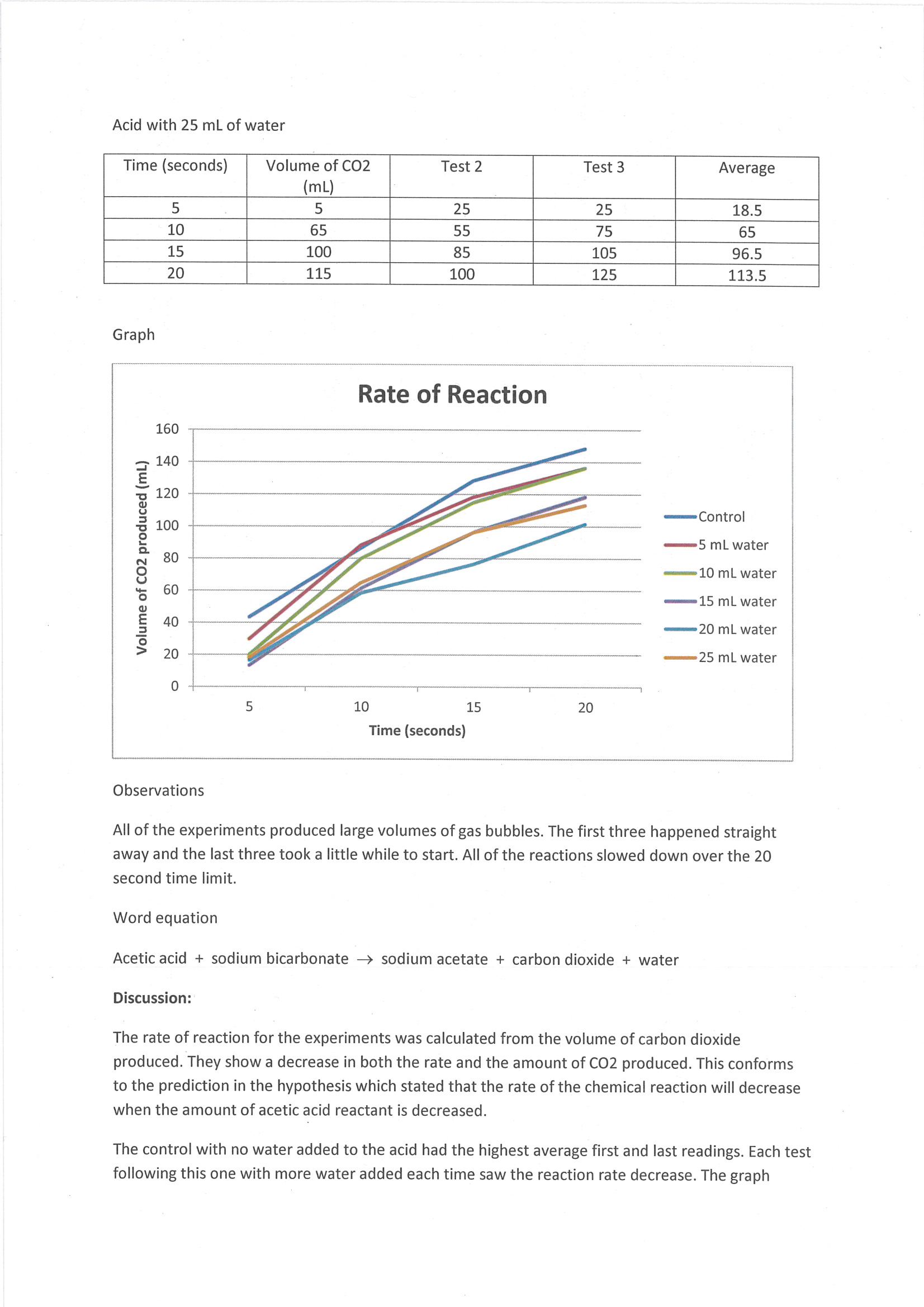
Constructs line graph to represent trends in results 2 Annotation 2
Includes qualitative observations that are relevant to discussion of results 3 Annotation 3
States word equation of chemical reaction investigated 4 Annotation 4
Describes trend in results
-
Annotations
-
1
Annotation 1
Constructs line graph to represent trends in results -
2
Annotation 2
Includes qualitative observations that are relevant to discussion of results -
3
Annotation 3
States word equation of chemical reaction investigated -
4
Annotation 4
Describes trend in results
 1
Annotation 1
1
Annotation 1
Identifies outlier in data and suggests human error as possible reason for uncertainty 2 Annotation 2
Constructs evidence-based argument that results are consistent with collision theory 3 Annotation 3
Reflects on validity of investigation and explains how other factors affecting reaction rate were kept constant 4 Annotation 4
Reflects on reliability of results 5 Annotation 5
Discusses possible sources of experimental error 6 Annotation 6
Suggests improvements to experimental method that would reduce or eliminate mentioned experimental errors 7 Annotation 7
Concludes that results support hypothesis and are consistent with collision theory while demonstrating misconception about nature and use of ‘proof’ in science
-
Annotations
-
1
Annotation 1
Identifies outlier in data and suggests human error as possible reason for uncertainty -
2
Annotation 2
Constructs evidence-based argument that results are consistent with collision theory -
3
Annotation 3
Reflects on validity of investigation and explains how other factors affecting reaction rate were kept constant -
4
Annotation 4
Reflects on reliability of results -
5
Annotation 5
Discusses possible sources of experimental error -
6
Annotation 6
Suggests improvements to experimental method that would reduce or eliminate mentioned experimental errors -
7
Annotation 7
Concludes that results support hypothesis and are consistent with collision theory while demonstrating misconception about nature and use of ‘proof’ in science
Above satisfactory
Rates of reaction
 1
Annotation 1
1
Annotation 1
States word equation as well as balanced molecular equation of chemical reaction investigated 2 Annotation 2
Identifies factors that influence rate of chemical reactions, including temperature, concentration, surface area and presence of catalyst 3 Annotation 3
States principal hypothesis of collision theory 4 Annotation 4
Uses scientific reasoning based on understanding of kinetic molecular theory to explain effect of temperature on reaction rate 5 Annotation 5
Uses mathematical reasoning to explain effect of concentration on reaction rate 6 Annotation 6
States essential nature of a catalyst, as relevant to collision theory, and uses deductive reasoning to explain its effect on reaction rate 7 Annotation 7
Makes clear prediction that is consistent with, and refers to, given explanation of collision theory
-
Annotations
-
1
Annotation 1
States word equation as well as balanced molecular equation of chemical reaction investigated -
2
Annotation 2
Identifies factors that influence rate of chemical reactions, including temperature, concentration, surface area and presence of catalyst -
3
Annotation 3
States principal hypothesis of collision theory -
4
Annotation 4
Uses scientific reasoning based on understanding of kinetic molecular theory to explain effect of temperature on reaction rate -
5
Annotation 5
Uses mathematical reasoning to explain effect of concentration on reaction rate -
6
Annotation 6
States essential nature of a catalyst, as relevant to collision theory, and uses deductive reasoning to explain its effect on reaction rate -
7
Annotation 7
Makes clear prediction that is consistent with, and refers to, given explanation of collision theory
 1
Annotation 1
1
Annotation 1
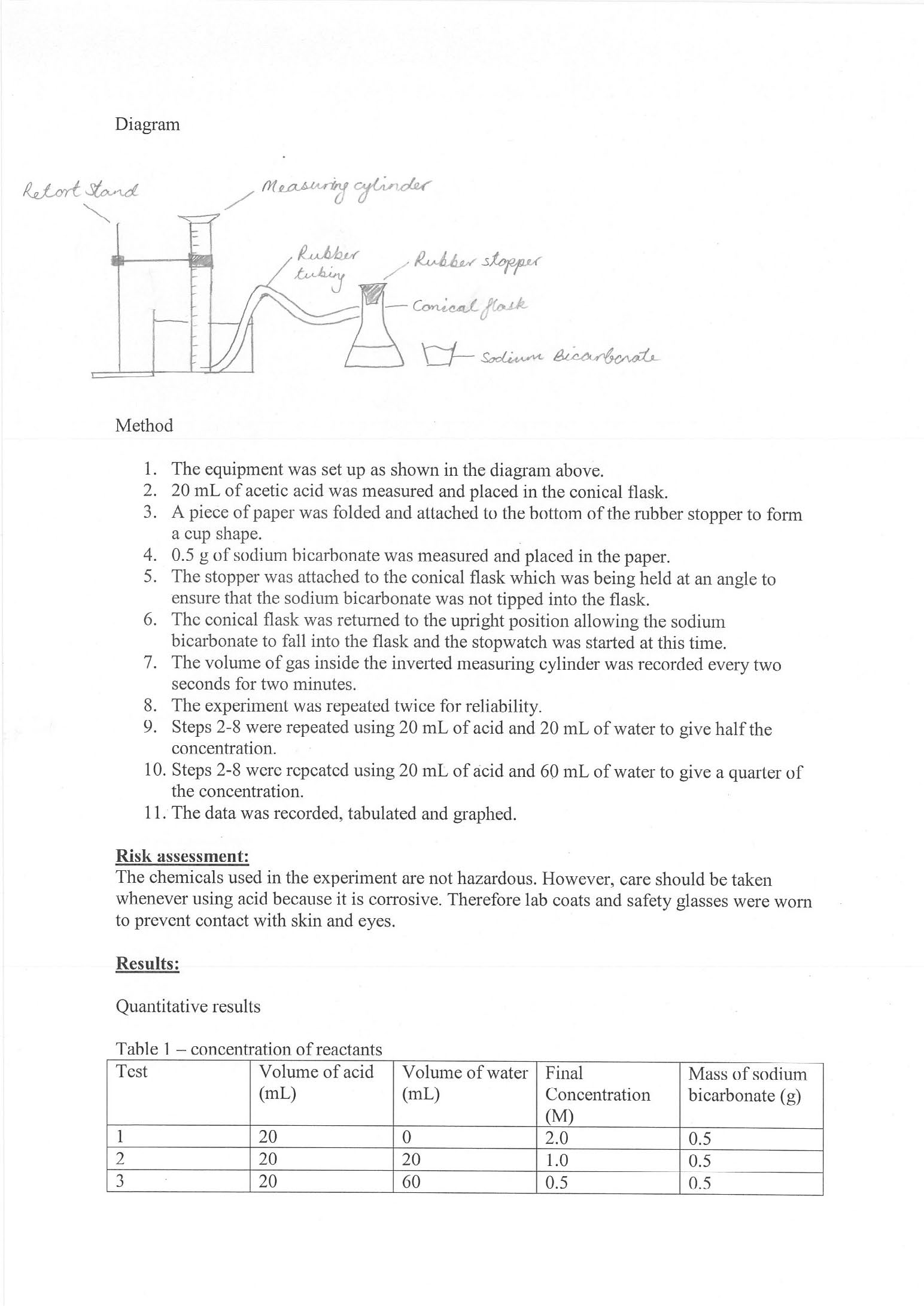
Provides diagram of experimental setup 2 Annotation 2
Designs logical and appropriate investigation method 3 Annotation 3
Considers reliability by performing repeated trials 4 Annotation 4
Specifies acid concentration as independent variable 5 Annotation 5
Considers safety precautions by specifying protective clothing and eyewear
-
Annotations
-
1
Annotation 1
Provides diagram of experimental setup -
2
Annotation 2
Designs logical and appropriate investigation method -
3
Annotation 3
Considers reliability by performing repeated trials -
4
Annotation 4
Specifies acid concentration as independent variable -
5
Annotation 5
Considers safety precautions by specifying protective clothing and eyewear
 1
Annotation 1
1
Annotation 1
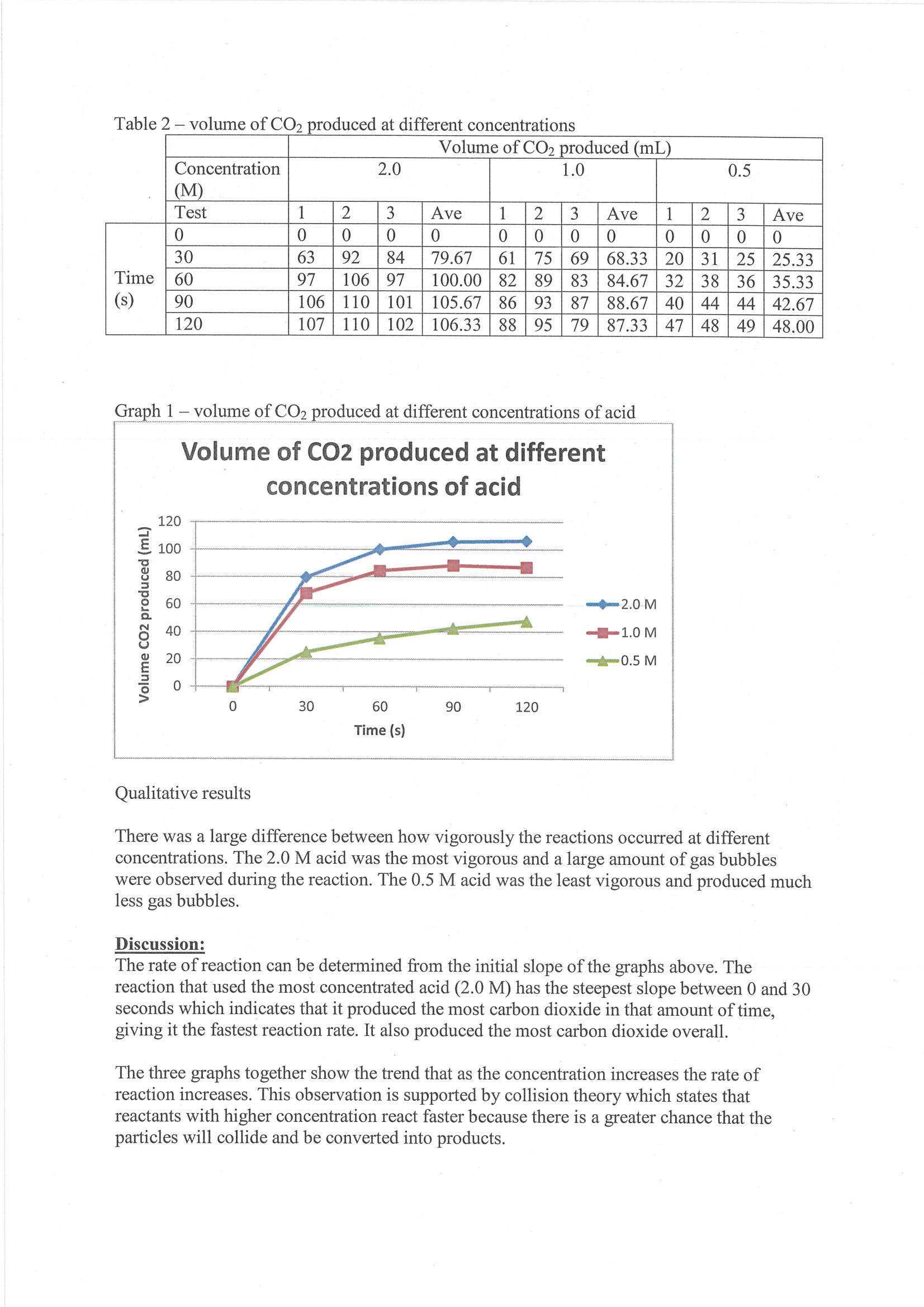
Presents raw data and averages in tabulated form 2 Annotation 2
Constructs line graph to represent trends in results 3 Annotation 3
Includes qualitative observations that are relevant to discussion of results 4 Annotation 4
Demonstrates scientific understanding of chemical kinetics by linking reaction rate to initial slope of curve 5 Annotation 5
Uses mathematical understanding of rates of change to interpret results 6 Annotation 6
Constructs evidence-based argument that results are consistent with collision theory
-
Annotations
-
1
Annotation 1
Presents raw data and averages in tabulated form -
2
Annotation 2
Constructs line graph to represent trends in results -
3
Annotation 3
Includes qualitative observations that are relevant to discussion of results -
4
Annotation 4
Demonstrates scientific understanding of chemical kinetics by linking reaction rate to initial slope of curve -
5
Annotation 5
Uses mathematical understanding of rates of change to interpret results -
6
Annotation 6
Constructs evidence-based argument that results are consistent with collision theory
 1
Annotation 1
1
Annotation 1
Reflects on reliability of results and identifies outliers as possible source of uncertainty 2 Annotation 2
Reflects on validity of investigation and identifies temperature as the only factor that may not have been perfectly controlled 3 Annotation 3
Suggests alterations to experimental method, including use of digital technologies, that would improve the reliability of results 4 Annotation 4
Provides list of appropriate sources that were used to research theoretical concepts underpinning investigation
-
Annotations
-
1
Annotation 1
Reflects on reliability of results and identifies outliers as possible source of uncertainty -
2
Annotation 2
Reflects on validity of investigation and identifies temperature as the only factor that may not have been perfectly controlled -
3
Annotation 3
Suggests alterations to experimental method, including use of digital technologies, that would improve the reliability of results -
4
Annotation 4
Provides list of appropriate sources that were used to research theoretical concepts underpinning investigation
Satisfactory
Rates of reaction
 1
Annotation 1
1
Annotation 1
States principal hypothesis of collision theory 2 Annotation 2
Identifies factors that influence rate of chemical reactions, including temperature, concentration and surface area 3 Annotation 3
Uses mathematical reasoning to explain effect of concentration on reaction rate 4 Annotation 4
Outlines experimental design specifying acid concentration as independent variable 5 Annotation 5
Makes prediction that is consistent with collision theory while demonstrating misconception about nature of ‘evidence’ 6 Annotation 6
Designs appropriate investigation method 7 Annotation 7
Considers reliability by performing repeated trials 8 Annotation 8
Considers safety precautions by specifying protective clothing and eyewear
-
Annotations
-
1
Annotation 1
States principal hypothesis of collision theory -
2
Annotation 2
Identifies factors that influence rate of chemical reactions, including temperature, concentration and surface area -
3
Annotation 3
Uses mathematical reasoning to explain effect of concentration on reaction rate -
4
Annotation 4
Outlines experimental design specifying acid concentration as independent variable -
5
Annotation 5
Makes prediction that is consistent with collision theory while demonstrating misconception about nature of ‘evidence’ -
6
Annotation 6
Designs appropriate investigation method -
7
Annotation 7
Considers reliability by performing repeated trials -
8
Annotation 8
Considers safety precautions by specifying protective clothing and eyewear
 1
Annotation 1
1
Annotation 1
Constructs line graph to represent trends in results 2 Annotation 2
Includes qualitative observations that are relevant to discussion of results 3 Annotation 3
States word equation of chemical reaction investigated 4 Annotation 4
Describes trend in results
-
Annotations
-
1
Annotation 1
Constructs line graph to represent trends in results -
2
Annotation 2
Includes qualitative observations that are relevant to discussion of results -
3
Annotation 3
States word equation of chemical reaction investigated -
4
Annotation 4
Describes trend in results
 1
Annotation 1
1
Annotation 1
Identifies outlier in data and suggests human error as possible reason for uncertainty 2 Annotation 2
Constructs evidence-based argument that results are consistent with collision theory 3 Annotation 3
Reflects on validity of investigation and explains how other factors affecting reaction rate were kept constant 4 Annotation 4
Reflects on reliability of results 5 Annotation 5
Discusses possible sources of experimental error 6 Annotation 6
Suggests improvements to experimental method that would reduce or eliminate mentioned experimental errors 7 Annotation 7
Concludes that results support hypothesis and are consistent with collision theory while demonstrating misconception about nature and use of ‘proof’ in science
-
Annotations
-
1
Annotation 1
Identifies outlier in data and suggests human error as possible reason for uncertainty -
2
Annotation 2
Constructs evidence-based argument that results are consistent with collision theory -
3
Annotation 3
Reflects on validity of investigation and explains how other factors affecting reaction rate were kept constant -
4
Annotation 4
Reflects on reliability of results -
5
Annotation 5
Discusses possible sources of experimental error -
6
Annotation 6
Suggests improvements to experimental method that would reduce or eliminate mentioned experimental errors -
7
Annotation 7
Concludes that results support hypothesis and are consistent with collision theory while demonstrating misconception about nature and use of ‘proof’ in science